Providing a High Professional Pharmaceutical Services and Care through Ensuring Sufficient Supply of Pharmaceuticals which are safe, Efficacious and of Acceptable Quality to the Population of Brunei Darussalam.
PRODUCTS CONTAMINATED WITH SCHEDULED POISONS, WESTERN MEDICINES, AND UNDECLARED CONTROLLED SUBSTANCES
These products are banned in Brunei Darussalam due to contamination with scheduled poisons, western medicines, or undisclosed controlled substances. The Ministry of Health prohibits their importation, sale, and use. Offenders can face fines up to $8,000 or six months’ imprisonment for selling such products under the Poisons Act 1956. If negligence causes harm, fines can reach $16,000 with twelve months’ imprisonment. Consumers are urged to stop using these products immediately and seek medical help if experiencing adverse effects.
Cosmetic products are notified based on the information declared by the importers. Importers of cosmetic products are responsible for the safety and quality of their products. Cosmetic products should not contain adulterants or prohibited substances/ingredients. Cosmetic products containing restricted substances/ingredients must comply with the limits and conditions of use of the cosmetic product.
Pharmacy Collection Service
Pharmacy AutoRefill Service – New content to describe medicine collection via AutoRefill Service
Easy Collect Service – New content to describe EC Service, details and TPOR
Pharmacy Delivery Service
New content to describe Pharmacy Delivery Services via GoRUSH, Postal Services and ONZ
Understand your medicines
New content – Pharmacy to come up with contents to assist public with regards to knowing their medicines
Medicines Use Review Service – meet with your pharmacist
Reporting Side Effects
New Content – to include description of what side effects are and how to go about reporting side effects and seek medical attention
Database for Import License And Wholesaler’s License (Updated December 2025)
The medicinal products manufactured, sold, supplied or imported into Brunei Darussalam are regulated under the Medicines Order, 2007 and its related regulations.
The Brunei Darussalam Medicines Control Authority (BDMCA) through the Department of Pharmaceutical Services is the Authority responsible for making decisions and recommendations in relation to any matter related to any registration, the issuance of any licences or the listing of any medicinal product.
Since mandatory implementation of drug registration system in July 2012, the pharmaceutical importers, wholesalers and manufacturers must register a medicinal product before the product can be marketed in Brunei Darussalam.
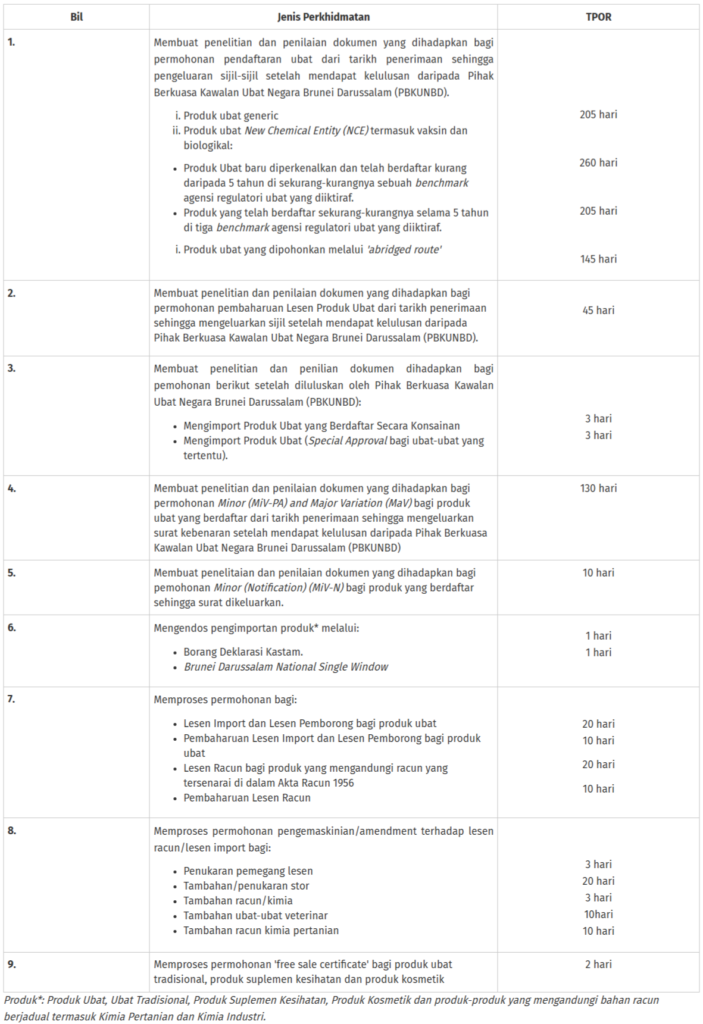
TPOR
How to reach us:
Drug Registration Unit, Product Regulation Section,
Department of Pharmaceutical Services, 2nd Floor, Spg 433,
Building of the Department of Pharmaceutical Services,
Kg Madaras, Rimba Highway, Brunei Darussalam.
+673 2393298 / 2393301 ext 225
drugregistration@moh.gov.bn
drugvariation@moh.gov.bn
OPENING HOURS
Monday – Thursday & Saturday
8:00 am – 11:45 am
1:45 pm – 4:00 pm
Monday 8:00 am – 11:00 am for the submission of:
Tuesday 8:00 am – 11:00 am for the submission of:
Wednesday 8:00 am – 11:00 am for the submission of:
*Submission of Applications are made by appointment basis.
Cosmetic products in Brunei Darussalam are regulated by the Medicines (Cosmetic Products) Regulations, 2007, which is in line with the ASEAN Cosmetic Directive.
Cosmetic products can only be marketed after it has been notified to the Ministry of Health through an upfront declaration of compliance by the importer or manufacturer before placing the cosmetic product in the local market.
TPOR

How to reach us:
Cosmetic Unit, Product Regulation Section,
Department of Pharmaceutical Services, Ministry of Health,
2nd Floor, Department of Pharmaceutical Services Building,
Spg 433, Rimba Highway, Kg Madaras, Mukim Gadong ‘A’, BE4710, Brunei Darussalam.
+673 2393301 / 2393230 ext 218 / 219 / 225
Fax: +673 2393297
cosmetic@moh.gov.bn
OPENING HOURS
Submission of NEW Cosmetic Product Notification
Tuesday & Thursday
8:00 am – 11:00 am
Submission of RENEWAL of Cosmetic Product Notification
Monday & Wednesday
8:00 am – 11:00 am
Collection and payment of new notification acknowledgement letter
Monday – Thursday
8:00 am – 11:00 am
Collection of renewal applications
(Note: Applicants that have no submission and only intends to collect renewed notifications are highly encouraged to come in the afternoon to avoid overcrowding)
Monday – Thursday & Saturday
8:00 am – 11:45 am
2:00 pm – 4:00 pm
Traditional Medicine and Health Supplement are products under the provision of Medicine Order, 2007. Currently, the manufacture, importation and/or sale of Traditional Medicine and Health Supplement do not require licensing or pre-market authorization from the Brunei Darussalam Medicines Control Authority (BDMCA). The onus of responsibility to ensure the safety and quality of such product rests on the company (importers, manufacturer and dealers/sellers).
Department of Pharmaceutical Services facilitate the importation and sale of Traditional Medicine and Health Supplement administratively in terms of quality, safety and in certain cases the efficacy of the product. Therefore, importers, manufacturers and dealers/sellers are encouraged to submit the application for importation and/or sale of Traditional Medicine and Health Supplement.
TPOR

How to reach us:
Traditional Medicine and Health Supplement Unit (TMHS Unit)
Product Regulation Section
Department of Pharmaceutical Services
Second Floor,
Building of the Department of Pharmaceutical Services
Spg 433, Kg Madaras, Rimba Highway,
Brunei Darussalam.
+673 2393298 / 2393301 ext 225
tmhs.unit@moh.gov.bn
OPENING HOURS
Submission of Application For Importation and/or Sale of Traditional Medicine and Health Supplement
Monday – Thursday & Saturday
8:00 am – 11:45 am
1:45 pm – 4:00 pm
© 2026 Ministry of Health Brunei. All Rights Reserved. Powered by Activ8 BN Digital Solutions
Get notified about new articles